Photoelectron Spectroscopy Explain the Difference Between Energy Levels
The maximum attainable signal-to-noise ratio is. In X-ray photoelectron spectroscopy the photoelectrons have energies characteristic of the atom they came from allowing us to make elemental and chemical determinations.
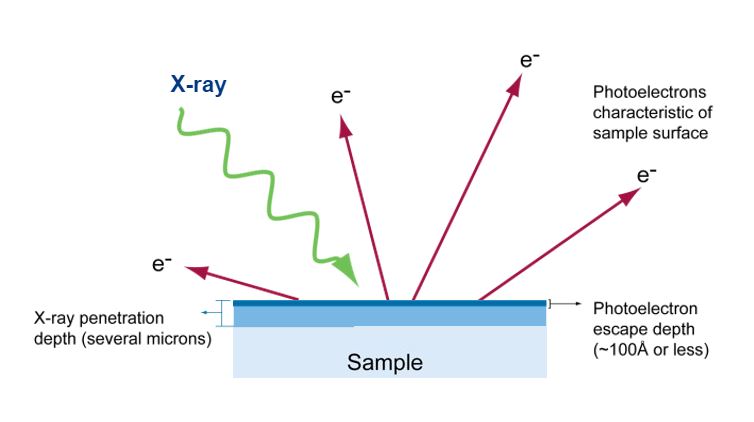
Xps Analysis X Ray Photoelectron Spectroscopy Or Esca
Of the secondary electrons is well below this.
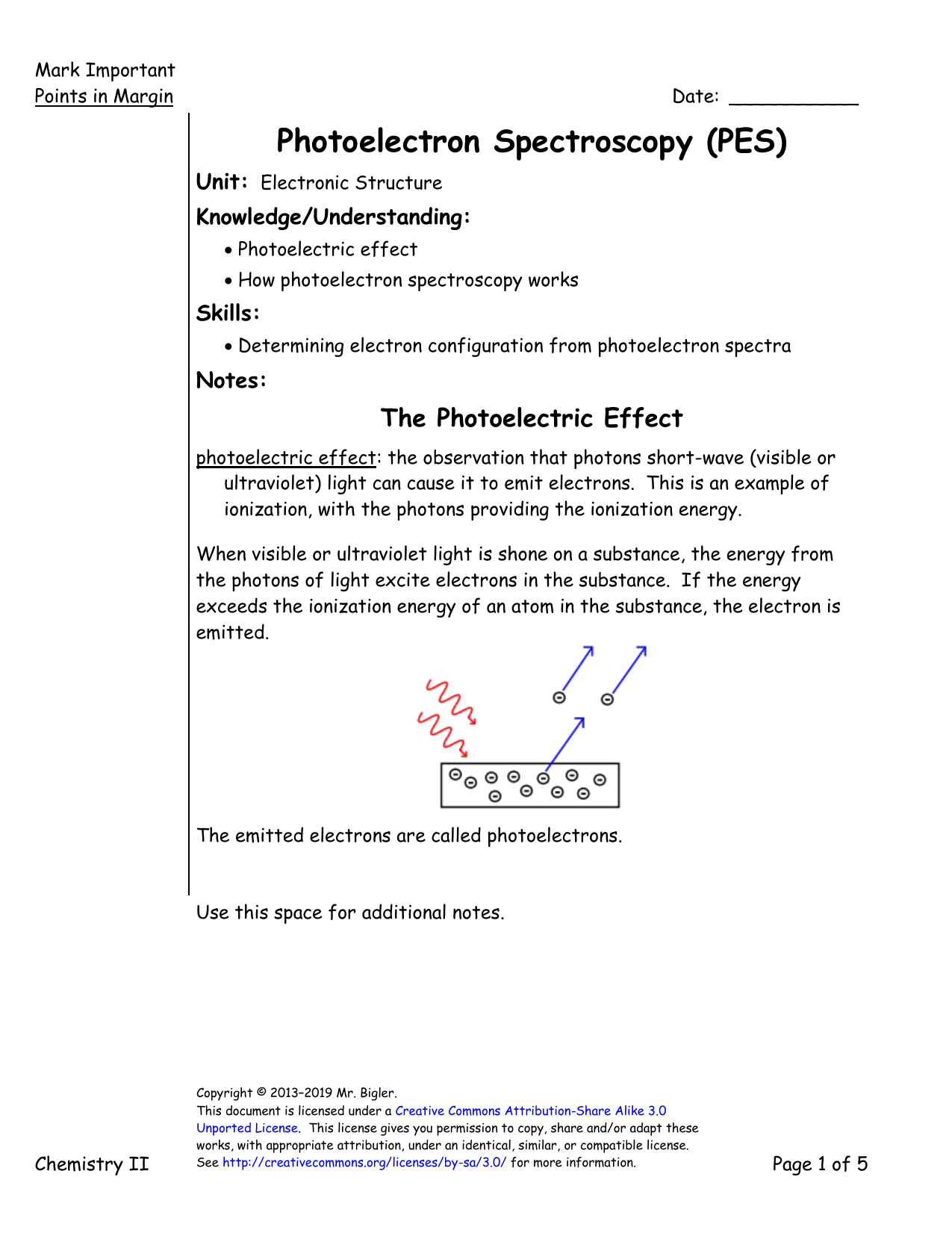
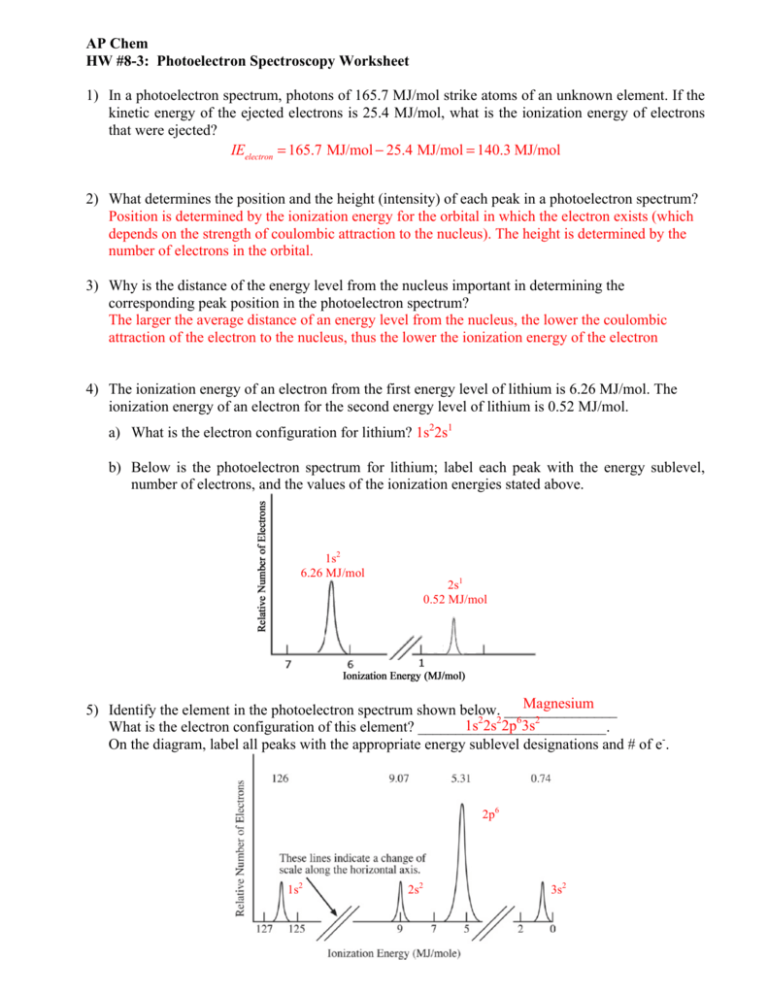
. The photoelectric effect first explained by Einstein in 1905 to atoms and molecules in all energy states. X-ray photoelectron spectroscopy XPS is a surface-sensitive quantitative spectroscopic technique based on the photoelectric effect that can identify the elements that exist within a material elemental composition or are covering its surface as well as their chemical state and the overall electronic structure and density of the electronic states in the material. In photoelectron spectroscopy one must compromise between energy resolution and the intensity of the detected signal S relative to the noise N.
KE h n - EA - EA The final term in brackets representing the difference in energy between the ionized and neutral atoms is generally called the binding energy BE of the electron - this then leads to the following. X-ray photoelectron spectroscopy XPS of five-monolayer-thick ZrO2 films reveals a core level binding energy difference of up to 18 eV between the tetragonal and monoclinic phase. Using either E hf E hcwavelength or for electrons E 12mv2 you can work out the energy of the incident photonelectron.
EA hv EA Ee- energy is conserved 2. Both quantities expressed in counts per second The resolution may be enhanced at a lower signal count rate and vice versa. An X-ray photon source is used in core-level spectroscopy while an ultraviolet source is used in.
Photoelectron Spectroscopy Utilizing anion photoelectron spectroscopy we are able to quickly determine the electron affinities and measure the vibrational frequencies of neutral molecules. Naturally all electrons will try and be as close to the nucleus as possible. The UPS measurements were performed in a multichamber EscaLab II system with a base pressure of the analyzer chamber in the lower 10 8 Pa range.
If this energy is equal to. Any photon energy in excess of that needed for ionization is carried by the outgoing electron in the form of kinetic energy. Photoelectron spectroscopy is based on Einsteins photoelectric effect.
The UPS spectra were recorded using HeI radiation photon energy E HeI 2122 eV generated in a differentially pumped windowless discharge lamp. One way to look at the overall photoelectron process is as follows. Learn how to interpret a photoelectron spectrum and relate it to the electron configuration of an element.
ESCA analysis which is also called X-Ray Photoelectron Spectroscopy or XPS uses an x-ray beam to excite atoms on the surface of a solid sample which results in. In previous chapters it was seen that absorption and emission of energy in the range 1 nm to 1000 nm corresponded to changes in electronic energy involving transitions between atomic or molecular electronic energy levels. KLL energy can be approximated as the difference between the C - 1s and valence levels minus the instruments work function typically in the 3 - to 5 - eV range.
Since the energy of the electron is present solely as kinetic energy KE this can be rearranged to give the following expression for the KE of the photoelectron. 1 A photon can ionize an electron from a molecule if the photon has an energy greater than the energy holding the electron in the molecule. Such transitions involved an initial electronic energy level and a final electronic energy level and the electromagnetic radiation absorbed or emitted in the.
Calculate the energy difference in Joules between energy levels. The BE of an electron is simply the difference between the initial state atom with n electrons and final state atom with n-1electrons ion and free photoelectron BE Efinaln-1 Einitialn If no relaxation followed photoemission BE - orbital energy which can be. In X-ray photoelectron spectroscopy XPS and Auger electron spectroscopy AES electrons emitted after the interaction between primary X-rays or electrons and a sample are detected.
The technique involves the bombardment of a sample with radiation from a high-energy monochromatic source and the subsequent determination of the kinetic energies of the ejected. In this technique the electrons are ejected from a packet of mass-selected anions A - by intersecting it with the harmonics of a NdYAG laser 1064 nm 532nm or 355 nm or by the. This difference is explained by positively charged oxygen vacancies in the tetragonal films which are slightly reduced.
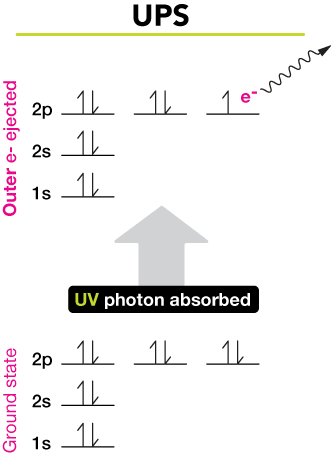
Pay careful attention to how electrons from the first energy level are different than electrons from the second energy level. Spectroscopy is the technique in chemistry where we measure the interactions between light energy photons and some molecule or molecular system. As indicated in Figure 5 photoelectrons originate from energy levels occupied by electrons including the valence band in inorganic semiconductors or HOMO energy levels for organic semiconductors as well as from core-level states which correspond to closed atomic shells.
The difference between the energy found in the bound state also known as the ground state and the vacuum level also known as the excited state is what we measure in photoelectron spectroscopy or PES. The energy of the electronic transition follows the state designation and symmetry information. So the key to working out if this is possible is.
These energy levels are quantized meaning that electrons can only reside inside the discrete levels not in between them. If the energy is equal to the difference in 2 energy levels the electron can be excited up 2 energy levels and so on. Conservation of energy then requires that.
Up to 24 cash back In order for a spectrum to be generated though you need a large sample of atoms so that electrons from all energy levels can be analyzed. Explore the analytical technique of photoelectron spectroscopy PES. A hv A e-1.
If youre seeing this message it means were having trouble loading external resources on our website. Since the electrons energy is present solely as kinetic energy KE this can be rearranged to give the following expression for the KE of the photoelectron. The main difference between the two emitted electrons is that the photoelectron is dependent on the irradiation energy while the Auger electron is only dependent on the energy difference between the orbital levels which is characteristic of each chemical element.
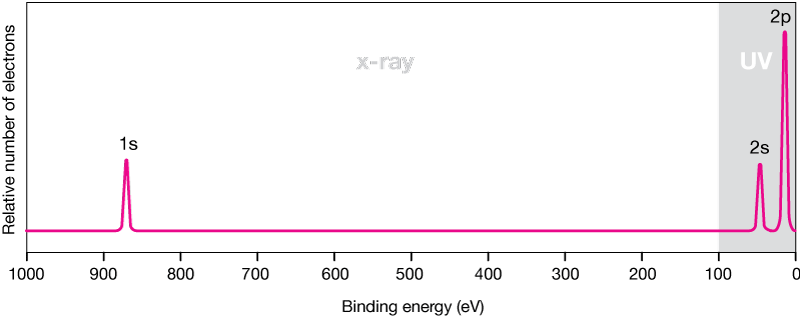
Of the C - 1s photo-electrons is well in excess of this 1200 eV and as stated in the question the KE. Ultraviolet photons used for UV photoelectron spectroscopy have lower energies and probe the electrons in the outermost valence levels of the surface atoms yielding surface electronic structure. Ultraviolet Photoelectron Spectroscopy.
So lets take a moment to look at the atomic level model. Photoelectron spectroscopy is an extension of the photoelectric effect see radiation. Where possible T 0 the energy separation between the electronic energy level of interest and the ground electronic vibrational and rotational states of the molecule is given.
However where only low resolution data or photoelectron data are available often only band maxima have been.
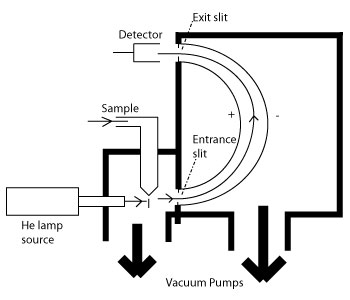
10 4 Photoelectron Spectroscopy Chemistry Libretexts

Interpreting Pes Data For Electron Configuration Youtube
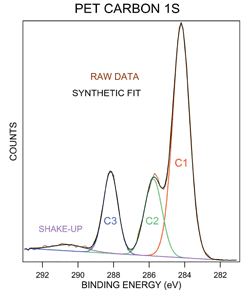
X Ray And Uv Photoelectron Spectroscopy Materials Science Nrel

The Photoelectron Spectroscopy Pes Youtube

13 Principle Of Photoelectron Spectroscopy Shown As An Example Is The Download Scientific Diagram

Photoelectron Spectroscopy Description Applications Video Lesson Transcript Study Com
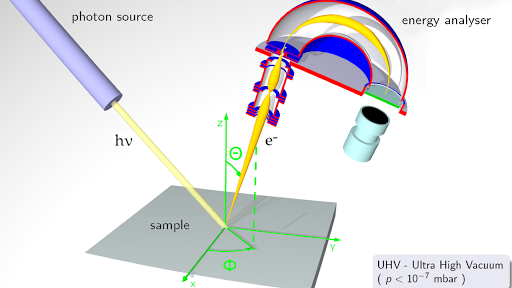
Photoelectron Spectroscopy Article Khan Academy
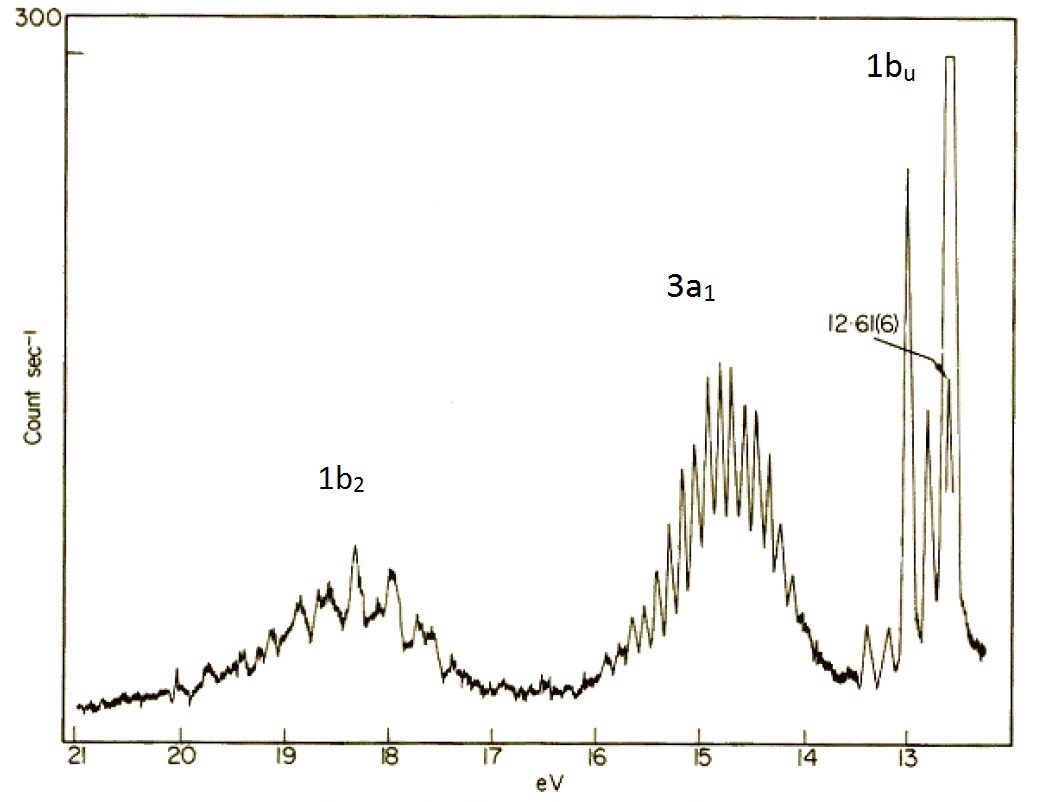
10 4 Photoelectron Spectroscopy Chemistry Libretexts

Photoelectron Spectroscopy Description Applications Video Lesson Transcript Study Com

Photoelectron Spectra Following Excitation Of The 2 P O 4p 3 D 1 Download Scientific Diagram

Photoelectron Spectroscopy An Overview Sciencedirect Topics
4 12 Photoelectron Spectroscopy Pes Ups Xps Esca Chemistry Libretexts
4 12 Photoelectron Spectroscopy Pes Ups Xps Esca Chemistry Libretexts

Comments
Post a Comment